Biomed’s strategy is to provide prevention and drug treatment in oral formulations to anyone who is at risks (such as healthcare providers, first responders) or patients in early onset of Covid-19 with the physician prescription.

Thanks to the polio vaccines, poliomyelitis has been eradicated globally, except Afghanistan and Pakistan are the only two countries where the disease is still classified as endemic continents. Prevention of polio disease spread has been accomplished by vaccination. There are two kinds of polio vaccine—oral polio vaccine (OPV), which uses weakened poliovirus, and inactivated polio vaccine (IPV), which is injected. The OPV is less expensive and easier to administer, and can spread immunity beyond the person vaccinated, creating contact immunity.
Oral polio vaccine has been approved by the FDA and more than 100 countries worldwide.
More than 1 billion people were vaccinated with OPV and effectiveness is 98%.
Vaccination for poliomyelitis is part of routine childhood vaccination. Either oral polio vaccine (OPV) or inactivated polio vaccine (IPV) have been administered in childhood inducing an immunity which, although protective for years, wans over time and is, for the most part, undetectable in adults unless a booster is administered. United States residents are advised to receive a booster prior to travelling to countries where polio disease remains endemic.
It has been found that an oral polio vaccine can be used as a prophylaxis early onset of Covid-19, because both poliovirus and coronavirus are positive-strand RNA viruses; therefore, they may induce and be affected by common innate immunity mechanisms.
Oral polio attenuated vaccines is more effective than IPV to fight against RNA viruses (including Poliovirus and coronavirus) inducing an immune response that recognizes the non-structural antigens of the viral particle. There is extensive homology between Poliovirus and SARS-CoV-2 RNA-dependent-RNA-polymerase (RdRp) both within the coding regions and illustrated in the 3-dimensional modeling. The homology between the viral epitopes may be sufficient such that adults who receive a polio booster develop an immune response that cross-reacts with SARS-CoV-2.
As a result, oral polio vaccine provides a powerful stimulant of an immediate, emergency response from the innate immune system against a wide range of other viruses including Covid-19.
One risk of using an oral polio vaccine is a potential rare side effect caused by vaccine-associated paralytic polio. The incidence was about 1 per million vaccinated children. The potential risks to adults would be extremely rare.
BioCovax™ is an oral polio vaccine which was formulated to be used in combination with Traneurocin, a neuroprotective agent.
Clinical trials results have demonstrated that NA-831 or Traneurocin, a neuroprotective drug can be administered orally in conjunction with oral polio vaccine to mitigate any potential risks of vaccine-associated paralytic polio.
BioCovax™ is an oral polio vaccine formulation which can be used as a prophylaxis for early onset of Covid-19 in adults.
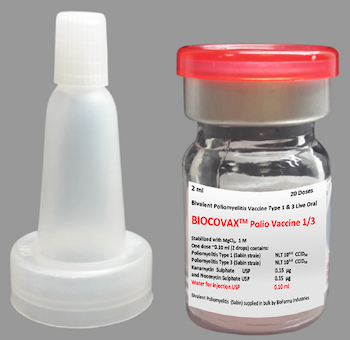
PRODUCT BROCHURE of BioCovax™ can be downloaded here.
DESCRIPTION
BioCovax™ B1/3 contains suspension of live attenuated poliomyelitis type 1 and type 3 viruses (Sabin strain) prepared in Primary Monkey Kidney Cells. BioCovax fulfills WHO requirements for Bivalent Poliomyelitis Vaccine y 1 & 3, Live (oral). Each dose contains the following:

ADMINISTRATION
BioCovax B1/3 must be administered only orally to adults (18 years to 85 years of age) for prophylaxis of Covid-19. Two drops are delivered directly into the mouth of the vaccinee from the multi-dose vial by dropper or dispenser.
Care should be taken not to contaminate the multi-dose dropper with saliva of the vaccinee. Once opened, multi-dose vials should be kept refrigerated between +2 degree C and +8 degree C in all time, and the vaccine is good for use for up to 28 days after opening of the vial, as recommended by World Health Organization (WHO).
SAFETY and EFFECTIVENESS
CONTRAINDICATION
STORAGE
PRODUCT BROCHURE of BioCovax™ can be downloaded here.
ORDERING BIOCOVAX Vaccine
For further information about our products, please contact us. Contact us
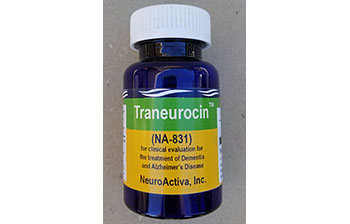
Traneuroci™ or NA-831 provides neuroprotection and catalyzing the regeneration of new neurons. Oral 30 mg per day for patients with early onset of Alzheimer’s Disease. NA-831 is manufactured by NeuroActiva, a subsidiary of Biomed.
Learn more
Biomed Digital is harnessing the power of wearable technology and machine learning to create a digital phenotype that helps clinicians to diagnose early onset of Alzheimer’s disease.
Learn more
Biomed is conducting Phase 3 clinical trials of NA-831 for the treatment and prevention of Alzheimer’s disease. We also conduct Phase 2/3 trials of NA-831 in combination with Atazanavir, Remdesivir, and Dexaneurosone and Ivermectin for treating Covid-19
Learn more
Vineurocin (NA-704) is an injectable drug, to regenerate new neurons, and reducting of muscular spasticity, a strong candidate for treatment of Alzheimer’s disease and Parkinson’s disease. NA-704 can be administered with the MICROS. NA-704 is in the Phase 1 safety studies.
Learn more
Combination therapy of NA-831 and an antiviral drugs such as Atazanavir, Remdesivir (Gilead Sciences) and anti-inflammatory such as Dexaneurosone and Ivermectin can enhance the effectiveness of the treatment of Covid-19.
Learn more