Our drug treatment strategy is to develop neuroprotective interventions during the severe acute respiratory syndrome, and provide appropriate rehabilitation measures afterwards.
Biomed’s strategy is to provide prevention and drug treatment in oral or intranasal formulations to anyone who is at risks (such as healthcare providers, first responders) or patients in early onset of Covid-19 so they can take the medication themselves with the physician prescription.

Biomed has developed BioCovax™, which is comprised of an oral polio vaccine with our neuroprotective agent called NA-831. The oral polio vaccine has been approved by the FDA and widely used in the US until 2004 when the polio was declared eradicated in the country. BioCovax™ is an oral formulation which can be used as a prophylaxis and treatment of early onset of Covid-19.
For more information, please visit the BIOCOVAX™ web page.A product brochure of BIOCOVAX™ can be downloaded here.
Biomed has developed Biomedivir™, which is comprised of our neuroprotective agent called NA-831 and Atazanavir. Atazanavir was approved by the FDA for prevention and treatment of HIV/AIDS treatment, and is now a generic drug. Biomedivir™ is an oral formulation which can be used as treatment for mild and moderate of Covid-19 infections.
Biomed plans to launch the Phase 2/3 trials of Biomedivir™ in 2021.
Biomed has developed Nanomedivir™, which is comprised of NA-831 and Remdesivir, anti-viral drug in nanoparticle-based intranasal formulation. Remdesivir (made by Gilead Sciences has been approved by the FDA for emergency use of severe cases of Covid-19.
Biomed plan to launch the Phase 1 clinical trials of Nanomedivir™ in 2021.
In addition, Biomed has also developed Dexaneurosone™, which is comprised of NA-831 and Dexamethasone in both oral and intranasal formulation. Dexamethasone is an FDA anti-inflammatory drug has been on the market for some 60 years and is used to treat conditions including arthritis and severe asthma. DDexaneurosone™ could be used to treat early and moderate cases of Covid-19.
Biomed plan to launch the Phase 2/3 clinical trials of Dexaneurosone™ in 2021.
For further information about our products, please contact us. Contact us
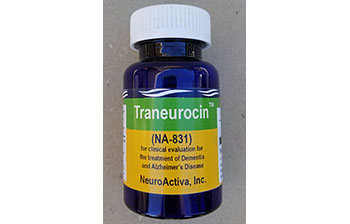
Traneuroci™ or NA-831 provides neuroprotection and catalyzing the regeneration of new neurons. Oral 30 mg per day for patients with early onset of Alzheimer’s Disease. NA-831 is manufactured by NeuroActiva, a subsidiary of Biomed.
Learn more
Biomed Digital is harnessing the power of wearable technology and machine learning to create a digital phenotype that helps clinicians to diagnose early onset of Alzheimer’s disease.
Learn more
Biomed is conducting Phase 3 clinical trials of NA-831 for the treatment and prevention of Alzheimer’s disease. We also conduct Phase 2/3 trials of NA-831 in combination with Atazanavir, Remdesivir, and Dexaneurosone and Ivermectin for treating Covid-19
Learn more
Vineurocin (NA-704) is an injectable drug, to regenerate new neurons, and reducting of muscular spasticity, a strong candidate for treatment of Alzheimer’s disease and Parkinson’s disease. NA-704 can be administered with the MICROS. NA-704 is in the Phase 1 safety studies.
Learn more
Combination therapy of NA-831 and an antiviral drugs such as Atazanavir, Remdesivir (Gilead Sciences) and anti-inflammatory such as Dexaneurosone and Ivermectin can enhance the effectiveness of the treatment of Covid-19.
Learn more